Automated Text Messaging for University, NIH-Funded and Cancer Center Research
Reminders, Intervention Information and Data Gathering Through a Single Platform
Every study is different, whether behavioral, public health or device research, each with its own unique elements or protocol requirements. Whether you are looking for participants to receive time-specific information, show up for appointments or to fill out surveys for study data collection, we have you covered. We’ve helped research teams in studies for nutrition, AIDS, addiction, smoking cessation, teen violence, diabetes, breast cancer and more.
The Mosio platform was designed to offer our clients the flexibility to configure the system based on specific needs. The biggest compliment we get is “this is so much better than what we were using before!” and we take pride in making consistent upgrades to the system based on customer feedback.
Improve Community Outreach Methods
Effective public health research relies on the researchers’ ability to engage a large audience from recruitment through to data analysis. Mosio’s award-winning suite of services assists public health researchers with the ability to more efficiently find and interact with patients using either their own phones or those provided by sponsors. With Mosio’s Storyline Alerts™ feature, researchers are able to pre-schedule and send a series of messages with each participant starting as they are screened, providing ease-of-use for studies with rolling enrollment or longer term studies with participants starting in phases.
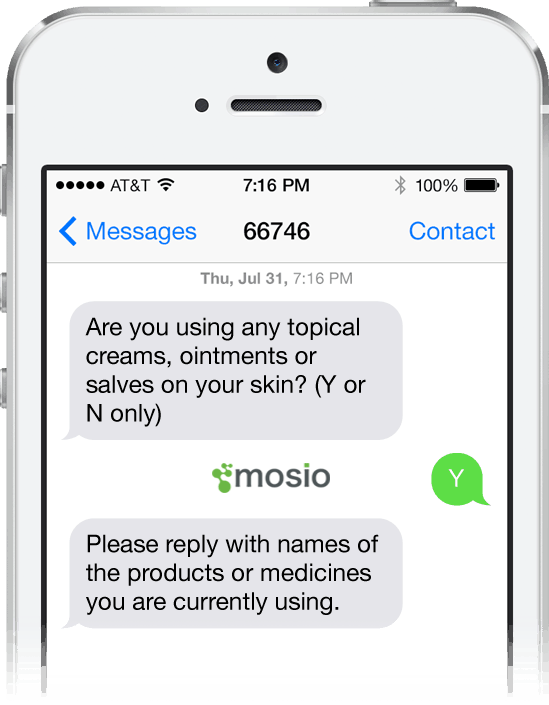
Storyline Alerts™ let you send surveys along with your messages, so you are able to test text-based intervention methods and collect data through the same process.

Efficient Communications Between Participants and Researchers
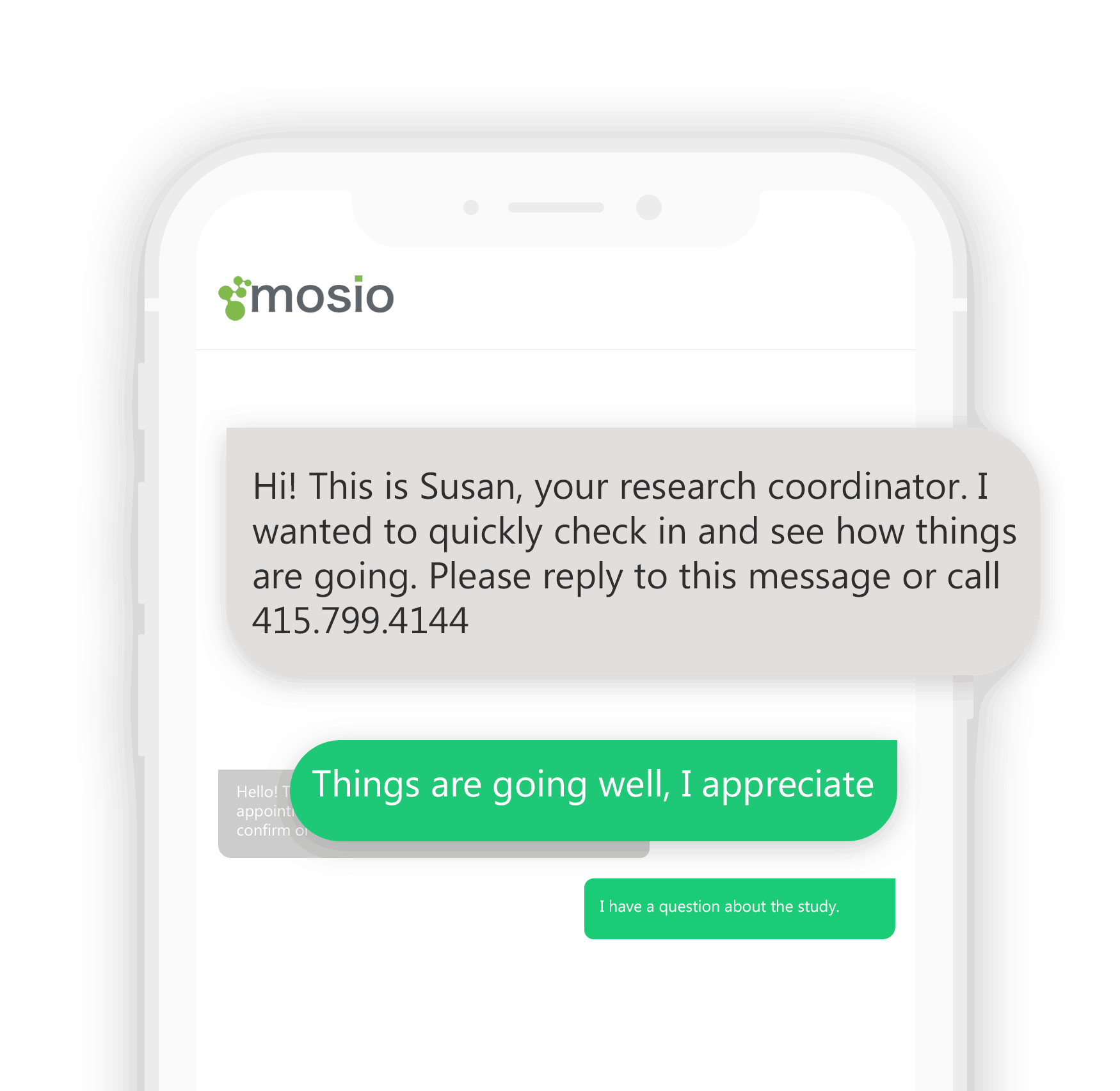
With the amount of patients being examined by researchers and the potential for cross-country research, Mosio’s Two-Way “TextChat” messaging is available as a real-time communication between patients and researchers, providing an easy platform for patients all over the country or world to contact the study site. Patients use their mobile phones, research teams use Mosio’s web-based software to receive and respond to text messages with a myriad of time saving features to make communications simple.
True Two-Way Text Messaging with International Reach
Mosio is available in over 50 countries where participants can send and receive questions and feedback as well as check in from a number within their country without paying international fees.
More Time for Data Analysis
Gathering clean data efficiently is one of the top reasons our public health clients want to use text messaging and use Mosio for their study needs. Surveys can be created and deployed within the Mosio system and our data export functions enable you to import CSV files into your preferred Electronic Data Capture (EDC) software for analysis. Mosio integrates directly with REDCap.
For those using paper journals or other data collection methods, Mosio is a great tool to ensure participants fill out their details within your specified time-frames and can be configured to provide them with a way to reply back to the message if they are confused or have additional questions about what is required.

