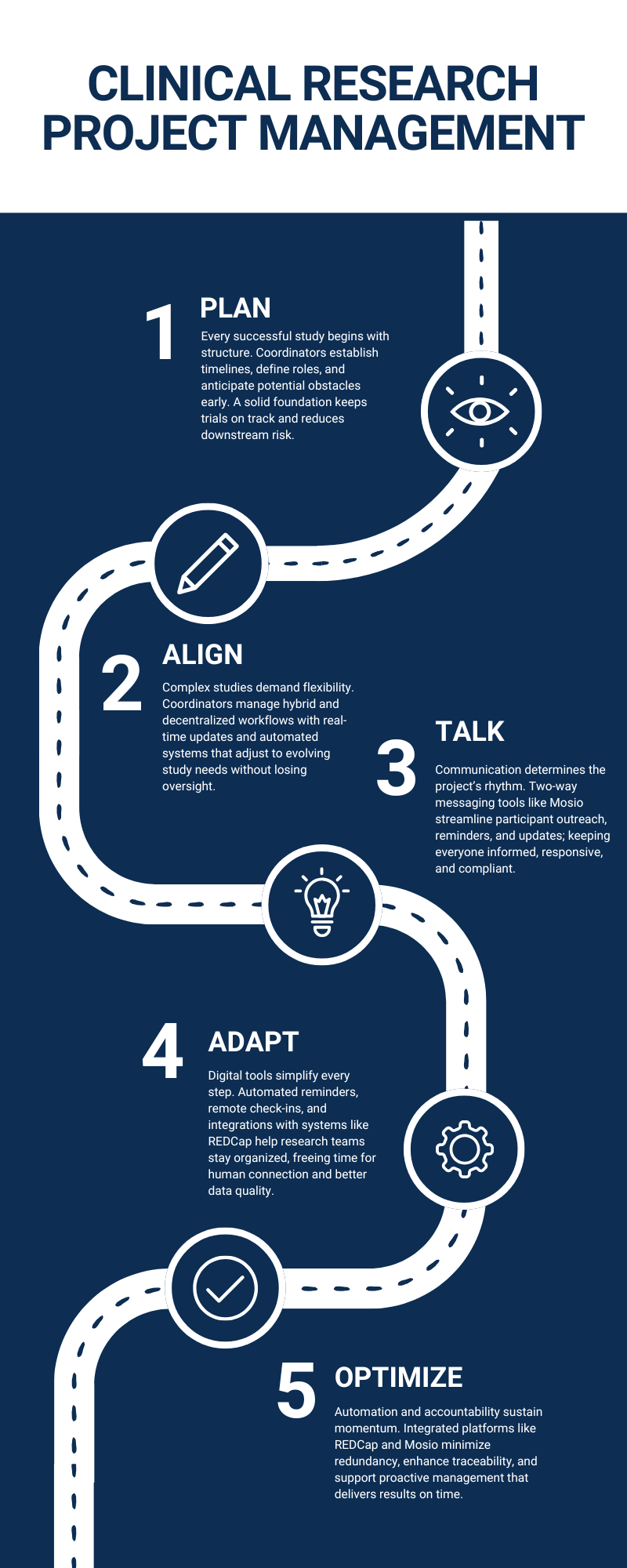
Clinical research project management decides the rhythm of every study.
We’ve talked about this quite a bit lately, including in our article on patient centricity, where we explored the idea of finding the “rhythm of a study.”
Deadlines, documentation, and coordination never align by accident. We know that structure shapes the outcome, and momentum keeps it alive.
When we speak with coordinators, they tell us that timing controls everything. Each step builds on the last. Delays in one corner echo through the entire system.
Understanding these challenges has enabled our team to create communication tools that keep pace with the speed of modern research, and that’s exactly why leading research organizations rely on our text messaging software.
Project management in clinical studies demands anticipation across several areas:
- Coordinators predict complications before they arrive.
- Data teams verify accuracy without slowing operations.
- Sponsors depend on steady handoffs.
The better the framework, the easier the trial.
Clinical Research Project Management Is All About Momentum
The best-managed clinical research projects operate on clarity. Every phase must align with:
- Specific goals
- Measurable outcomes
- Controlled communication channels
We’ve found that when roles are clearly assigned, teams move efficiently from planning to execution without unnecessary overlap.
Mosio delivers clarity through reliable communication workflows that help research staff organize outreach and scheduling. Coordinators receive tools that integrate naturally into existing systems, reducing time lost in fragmented communication.
What does this look like? An automated and interactive text messaging solution to improve adherence, engagement, and data collection in your studies.
How Do Coordinators Maintain Consistency Across Phases?
We know that consistency depends on three things:
- Documentation
- Real-time updates
- Shared visibility
A unified plan allows project leads to track recruitment, monitor progress, and evaluate performance against established benchmarks. Standard operating procedures support that rhythm.
We’ve seen study teams strengthen collaboration when they adopt structured messaging through Mosio. Clear, direct communication creates accountability and maintains study integrity. Participants respond faster, and site staff stay synchronized. This is why patient centricity is always a win-win.
Communication Will Determine Project Momentum
Every study advances at the pace of its communication. Delays often originate from unclear messages or missed follow-ups. This is why coordinators who prioritize communication will usually manage to avoid common slowdowns like scheduling conflicts or incomplete data entry.
What we’ve seen is that teams using centralized messaging platforms experience fewer disruptions. Messages reach participants immediately, updates are logged automatically, and coordination becomes effortless. Communication stops being a separate task and becomes part of the project’s natural flow.
Managing Risk During Complex Studies
Risk management requires foresight and ongoing assessment. Coordinators track potential disruptions related to compliance, recruitment, or logistics. Each variable receives attention early, before small issues evolve into study-wide challenges.
We’ve noticed that digital communication platforms reduce risk exposure through improved documentation and real-time traceability. You’ll see that every interaction leaves an auditable trail that supports regulatory confidence and operational reliability.
Planning creates room for adjustment. Coordinators use structured timelines that identify dependencies before they collide. When unexpected events occur, contingency paths keep studies aligned with deadlines.
Mosio’s automation features reinforce those plans through timed reminders and consistent participant engagement. Teams can shift priorities quickly without losing track of existing commitments. That balance prevents cascading delays and ultimately protects study integrity.
Technology Unites Every Moving Piece Of Research
REDCap structures the data. Mosio manages the flow of communication. Together, they establish an organized environment that minimizes errors and redundancies.
Automated notifications replace manual tracking, which allows teams to spend more time analyzing data and less time chasing updates. What you’ll find is that oversight becomes active, not reactive.
Some studies outperform others under similar conditions. Why? Usually, it comes down to discipline in project execution. When communication, scheduling, and documentation align, projects stay ahead of schedule. Every decision made early compounds into better outcomes later.
What we’ve seen is that consistent communication produces more reliable data and stronger relationships between teams and participants. Smooth coordination generates measurable advantages without additional resources.
How Are Teams Adjusting To Hybrid & Decentralized Trials?
Hybrid trials require flexible systems that bridge remote and in-person workflows. Coordinators adapt to multiple communication channels while keeping study data unified. Flexibility allows participants to engage without losing oversight.
We see Mosio’s platform functioning as the anchor in this transition. Two-way text messaging gives remote participants simple access to coordinators while maintaining compliance. That stability ensures reliable engagement regardless of study location.
It’s always interesting to observe motivation growing through progress visibility and consistent acknowledgment. Teams stay energized when they can measure advancement and witness real impact.
Generally speaking, coordinated communication reinforces team morale. Transparent updates and organized workflows prevent burnout. We’ve found that success feels tangible when results are tracked and shared across all collaborators.
Where Is Clinical Research Project Management Heading?
The most successful project management models will integrate automation with human expertise. Mosio is allowing teams to move faster, communicate better, and keep participants informed.
This means that every process becomes:
- Traceable
- Compliant
- Responsive
We know that clinical research will always depend on human coordination, and with the right infrastructure, that coordination creates manageable operations.
For us, this means continuing to provide research teams with software for creating automated text message programs that improve study adherence, engagement, and data collection.